Domperidone Test Purchase Exercise
Background
In October 2014, the PSI published updated guidance on the Safe Supply of Non-Prescription Medicinal Products Containing Domperidone to highlight the increased adverse cardiac risks associated with domperidone use identified by the European Medicines Agency (EMA) and to notify pharmacists of the updated marketing authorisation.
PSI Test Purchase Exercises
Considering these changes and the updated PSI Guidance, we have carried out test purchase exercises for the past three years in community pharmacies, selected at random, to check whether non-prescription medicines containing domperidone were being supplied in accordance with the updated guidance.
The PSI conducted a further test purchase exercise between December 2016 and January 2017. A total of 75 community pharmacies were visited in this mystery shopper exercise. While most pharmacies were selected at random, 13 were selected to be reassessed based on their failure to comply with the updated guidance during previous visits.
How was the test purchase carried out?
The test purchase scenario instructed the mystery shoppers to:
- Request a domperidone-containing non-prescription medicine,
- Purchase the product,
- Note any question(s) asked by the pharmacy staff members and interactions with the individuals engaged in the sale/supply of the medicine,
- If questions were asked of the mystery shopper, s/he was instructed to inform the pharmacist/other staff member that the medicines were for their partner or relative who has some nausea and bloating, who is 61 years old and is on tablets for their heart.
- Ask to speak to the pharmacist once the transaction was completed to verify whether the pharmacist made the sale or not. Based on this scenario, domperidone is not a suitable medicine for this patient and a sale should not have been carried out.
Findings
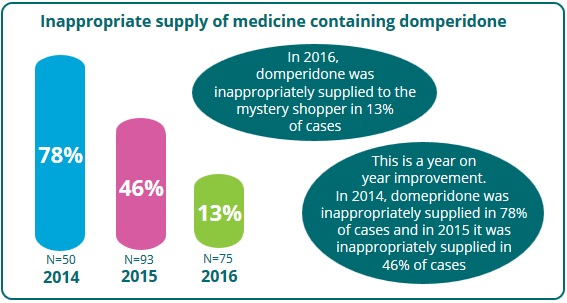
Specific comparison
Of the pharmacies visited in 2016, 17% were revisited after previously failing the exercise. None of these pharmacies supplied the domperidone-containing medicine during the revisit, and this is a positive outcome.
Who made the inappropriate supply?
In 2016, 50% of the inappropriate supplies were made by a pharmacist, and the remaining 50% by another pharmacy staff member.
Outcomes and Concerns
The exercise has been carried out to reinforce the importance of maintaining up-to-date knowledge of changes in the use of medicines, their contraindications and potential for harm to patients. The recent findings, and the previous years’ comparisons, indicate a positive improvement in compliance, and awareness raised about supply of domperidone containing products. While noting the improvements in relation to this type of medicine, the supply of medicines contrary to their marketing authorisations, and PSI guidance, remains a concern to the PSI.
- Consultation: Supplies of these products are being made where pharmacists are not carrying out a consultation, or a proper consultation, with the patient or their carer to ensure that the medicine is safe and appropriate for that person.
- Clinical Awareness: All pharmacists are not demonstrating full clinical awareness of the updated indications and contraindications for the use of domperidone.
- Staff Training: All non-pharmacist staff may not have been properly trained on the updated requirements of the marketing authorisation for domperidone and PSI guidance.
- Storage: Some pharmacies continue to inappropriately store non-prescription domperidone-containing medicinal products behind the medicines counter or in the public pharmacy area, instead of in the dispensary.
Advice
All pharmacists are reminded to ensure that they keep their knowledge up to date and ensure that they are familiar with the updated product information for medicines containing domperidone, including the requirements of the PSI guidance.
In addition, superintendent and supervising pharmacists should ensure that all staff members are aware that requests for domperidone must be referred to the pharmacist. The pharmacy’s SOPs should be updated to reflect these changes if this has not already been completed. Refer to the Guidance on the Safe Supply of Non-Prescription Medicinal Products Containing Domperidone for detailed information.
Note
All pharmacies that were included in the test purchase exercise between December 2016 and January 2017 will receive correspondence informing them of the outcome of the test purchase. The PSI may carry out further test purchase exercises to ascertain compliance in the supply of medicines to improve outcomes for patients.